Crystal Morphology and Lamellar Growth
Under certain conditions, polymers cooled from the melt arrange into regular crystalline structures. The fundamental structure of these polymer crystals is the lamella which consists of tightly packed and folded polymer chains (loops) assuming a mostly planar zigzag and helical configuration (polyolefins). In many cases, these lamellae form large spherical crystals called spherulites which have a dendrite-like structure with different degree of regularity.
The degree of crystallinity can vary between completely amorphous to almost entirely crystalline (95%). However, crystalline lamellae have typically a less perfect structure than crystals formed from low molecular weight compounds because of misalignment, kinking, twisting, etc. which prevents the complete ordering of every repeat unit of every polymer chain. Furthermore, the reentry of the folded polymer chains at the lamellar surface causes some disorder, because the polymers usually do not fold regularly along the growth plane. In many cases, the reentry of the polymer chains is completely random which creates a diffuse phase boundary. Only in a few cases, do polymer chains fold back into the lamellae in an ordered pattern. This growth mode is known as the adjacent re-entry model whereas the completely random non-adjacent reentry is called switchboard re-entry (see figure below). It is also possible that some parts of the chains remain in the amorphous phase or re-enter at a distant location or in other crystals.
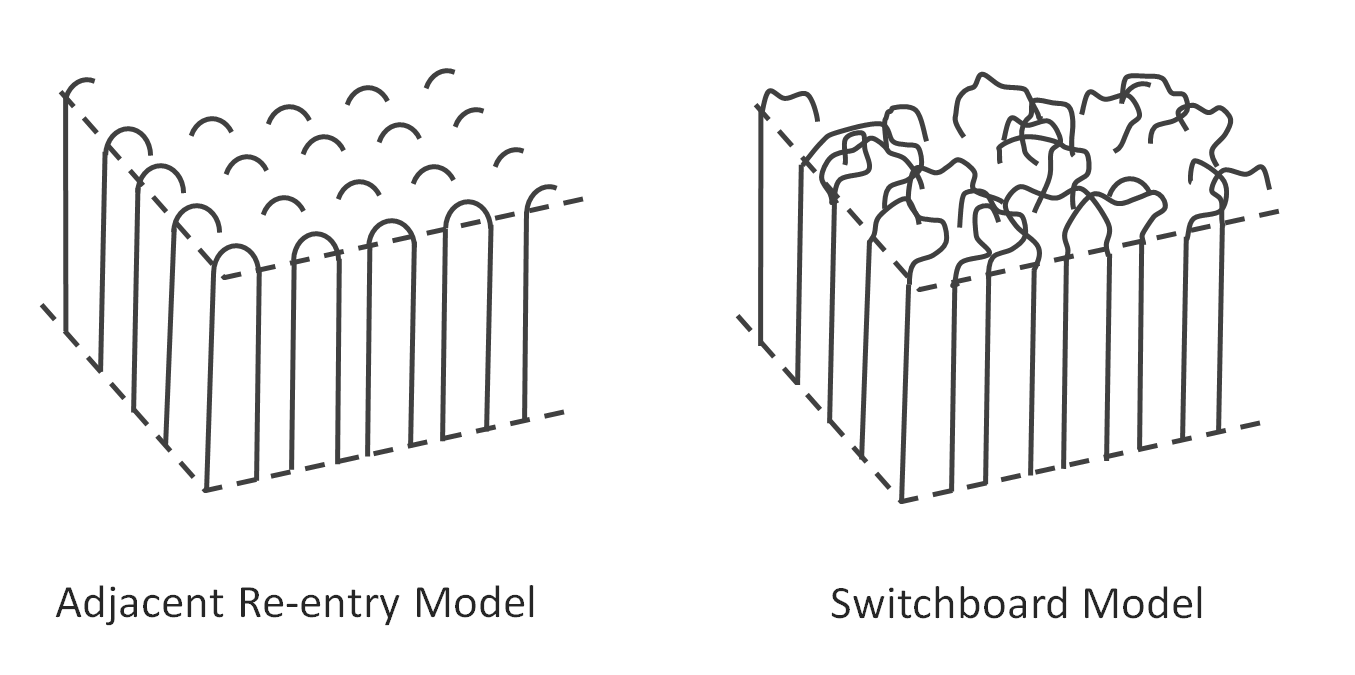
Very flexible polymers such as polyethylene will mainly re-enter the lamellae at adjacent sites when slowly crystallized whereas fast cooling will result in some random re-entry (at a distant location). Another small portion of the chain ends remains in the amorphous phase or enters another neighboring lamella. In many other cases, polymer crystal growth with adjacent re-entry is either not possible or very unlikely. For example, polymers with a very stiff backbone such as PEEK or PES are incapable of adjacent re-entry as these polymers cannot bend over a short distances, and hence, can only re-enter the crystal several stems away from the exit point, whereas very stiff polymers such as liquid crystals will predominantly enter other crystals and/or a large portion of the chains will remain in the amorphous phase.
Many polymers are not linear but include a certain degree of long or short chain branching which will effect the structure and degree of crystallinity. For example, short branches such as butyl, hexyl and octyl, which are purposefully introduced in linear low density polyethylene (LLDPE), reduce the overall crystallinity and the size of the spherulites whereas long branches can undergo side-chain crystallization. Branching also increases the amorphous portion in the spherulites because side chains disrupt/impede lamellar growth.